Clinical Trials Laboratory Services

Clinical Histopathology
Experienced on-site pathology team
The on-site certified pathology team led by Prof J. Rueschoff and Prof. B. Jasani is complemented by a large international network of renowned pathologists. Together they combine for expertise in all diagnostic indications, molecular and clinical pathology, biomarker analysis and case annotation for digital pathology. This allows for histopathology inspection of all samples.
- Six on-site pathologists plus 30 consultant pathologists
- Strong clinical and scientific track record
- Inter-observer harmonisation by internal and external proficiency testing
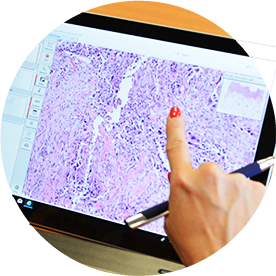
Digital Pathology
Pathologist annotation for algorithms in immune-phenotyping.
Digital scans of bright-field and fluoresent tissue slides are annotated by pathologists and undergo computer-assisted quantification after IHC or Immunofluorecence Staining our Digital Pathology Team has experience with several software platforms including AI algorithms.
- Various scanning platforms (Aperio, Roche, Zeiss)
- Digital immune-phenotyping with Visiopharm
- Spatial resolution and qualification of signals

DNA/RNA Isolation
10 years of experience in isolating and analyzing of DNA & RNA
Targos has extracted high quality nucleic acids from 50.000 global clinical samples with commercially available state-of-the-art extraction and analysis methods (e. g. Roche, Qiagen, NanoDrop). Manual and automated extraction protocols include tissue macrodissection to yield best quality and quantity for all purposes.
- Screening and batch protocols available
- RNA extraction for RNA Seq or Nanostring, DNA for NGS or mutation analysis
- Extraction services helped to approve the COBAS V600E B-Raf test

ELISA
Analyzing soluble serum and tissue markers.
Enzyme Linked Immunosorbent Assays (ELISA) have been used on a routine basis to monitor therapeutic response in clinical trials for quantitative determination of serum HER-2/neu or EGFR. In addition, we have also set up customized assays for detection of target protein phosphorylation in solubilized surrogate tissues, such as skin. Appropriate assays for sensitive Cytokine detection are currently evaluated.
- FFPE, Liquid Biopsies & Blood
- Soluble markers
- Phospho-Proteins & Cytokines

Expression Profiling
From fast turnaround CDx analysis to large transcriptome analysis.
Based on high quality. RNA extraction Targos has evaluated single gene expression and transcriptomes. Automated RNA in situ expression analysis was applied in approval trials (RNAscope).
- Nanostring & RNA Seq
- in situ RNAscope
- qRT-PCR

Histology
Tissue feels home at Targos.
All general histology services such as paraffin embedding or preparation of frozen tissues; H&E staining (using HE600 and Varistain platfoms); sectioning (under standard or RNAse-free conditions) and under GCP/ GLP conditions. Turn-around time is one day for small batches (n= < 10).
- Very flexible protocols for any slide assignment
- Special protocols to serve digital pathology needs
- Constant feedback from pathologists to technicians
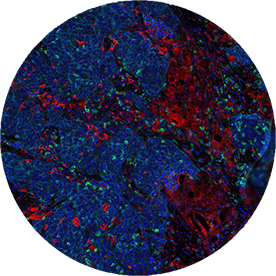
Immunofluorescence (IF)
Immune phenotyping in clinical trials.
Biomarker testing for targeted therapies by IHC has evolved into multiplex IF to address response phenotyping for immune therapies. Targos uses expertise in IHC to validate multiplex IF for application in clinical trials.
- Offering several panels with sensitive and robust Ultivue technology
- Evaluation of assays with Visiopharm Apps
- Co-Development of novel IF Panels with Ultivue
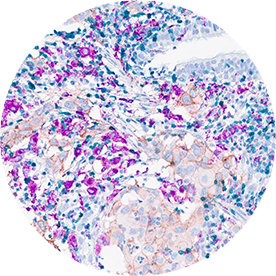
Immunohistochemistry (IHC)
Core technology since the 2005.
Targos has performed IHC services in over 500 clinical trials and in many preclinical projects. Our state-of-the-art IHC labs have applied more than 200 different assays for many tissue-based biomarkers. Development of novel evaluation algorithms are our core expertise, such as HercepTest for gastric cancer. Immune-response monitoring with Multiplex-IHC led to digital pathology evaluation since 2015.
- Ventana, DAKO & Leica platforms available
- > 200 IHC single plex assay validated
- Immune oncology multiplex assays on Ventana Discovery (e.g. CD4, CD8, PD-L1, PD, CD68, FOXP3, panCK, Ki67)

In Situ Hybridization (ISH)
Full range of manual and automated interphase ISH assays.
All assays are evaluated by board-certified pathologists . This includes FDA-approved bright-field and fluorescence assays from Abbott Molecular, Dako-Agilent and Roche-Ventana. We also develop custom assays based on customer requests. Turn-around time is usually two business days for small batch assays (n= < 10 ). For larger batches and service in the context of archiving or shipment of samples, please contact us at info@targos-gmbh.de.
- Approval of six ISH assays supported
- > 10.000 ISH assays analyzed
- Inter-observer harmonisation by internal and external proficiency testing
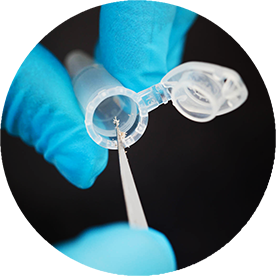
Mutation Analysis
Turn-around time of 3-5 business days.
Several years of in house assay development and validation experience enable Targos to produce accurate, precise and specific results. Our assays are optimized to work on ‘real life’ patient samples and have low drop-out rates. Currently, we offer commercially available tests on the Roche Cobas® 4800 platform and on QIAGEN's Rotor-Gene Q. Custom assays can also be developed upon request.
- Analysis of 7000+ B-Raf assays with TAT of <5 days led to CDX approval
- 3000 PI3K assays within 3 month
- Single sample analysis can be accomodated for screening

PCR
Turn-around time of 3-5 business days.
Targos offers “real-time reverse-transcription polymerase chain reaction” ( qRT-PCR) service for simultaneous amplification and quantitation of targeted DNA using state-of-the-art thermocyclers , gel electrophoresis and documentation equipment.
- FFPE, Liquid Biopsies & Blood

Sequencing
Multiple protocols to unravel sequence form clinical samples.
The biggest challenge for nucleic assay sequencing in clinical research is the preparation of best quality and quantity templates from tissue samples.
With our expert clinical partner labs in Cologne and Essen, Targos is able to provide selected solutions for panel sequencing for RNA Seq, DNA mutational and gene fusion analysis on Illumina NextSeq and MySeq platforms for your translational clinical projects.
- FFPE, Liquid Biopsies & Blood
- TruSight 500 /TMB panels
- RNA Seq
- Avenio panels
